ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Chemical Reaction Rates Videos 10 videos
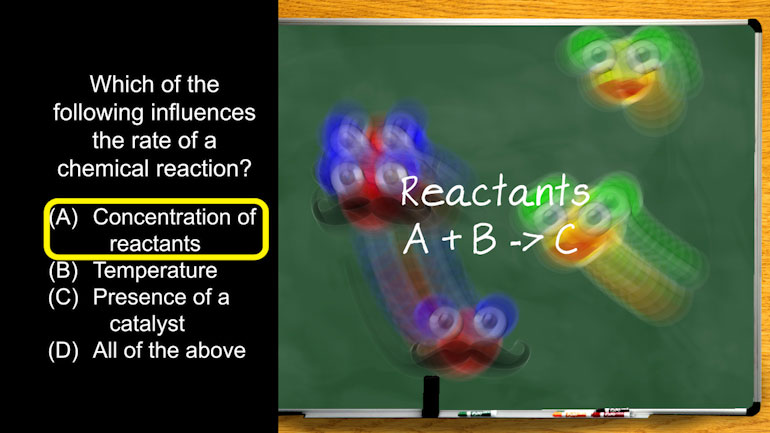
AP® Chemistry: Reaction Rates Drill 1, Problem 1. Which of the following influences the rate of a chemical reaction?
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 3.1 Chemical Reaction Rates 3 Views
Share It!
Description:
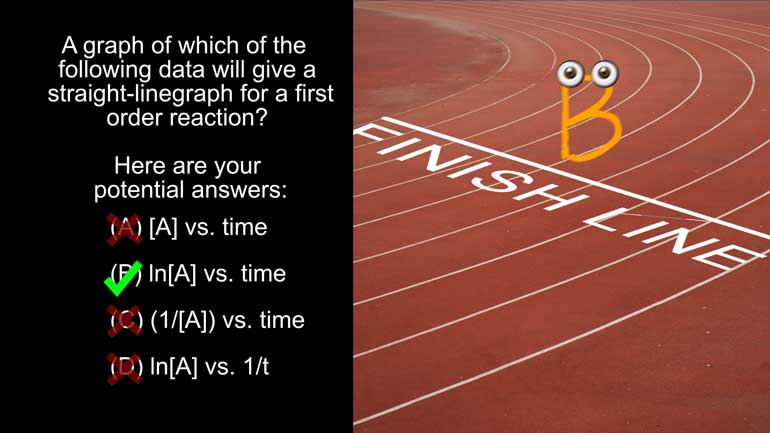
AP Chemistry 3.1 Chemical Reaction Rates. A graph of which of the following data will give a straight line graph for a first order reaction?
Transcript
- 00:03
Here’s your Shmoop du jour, brought to you by lines, helping scientists get dates since
- 00:08
1811. [Boy and girl looking at paintings]
- 00:09
Because what's dating without a little…chemistry?
- 00:12
Alright, here’s today’s question:
- 00:14
A graph of which of the following data will give a straight-line graph for a first order
Full Transcript
- 00:18
reaction?
- 00:19
And here are your potential answers: All right, so we know that for a first order
- 00:25
reaction, the rate is proportional to the concentration of the reactant. [First order reaction equation]
- 00:29
That means that as the reaction proceeds, the concentration of the reactant decreases
- 00:33
more and more slowly.
- 00:35
To be a little more specific, the concentration of [A] decays exponentially.
- 00:40
Just like your concentration in the math class you have right before lunch. [Students in math class]
- 00:44
If your eyes are in proper working order, you can tell by looking at the graph that
- 00:47
this is not a straight line. [Graph with a sloping line]
- 00:49
If it looks like one to you, we highly recommend a quick visit to the optometrist. [Girl conducting an eye-exam]
- 00:52
Anyway, since this is a graph of the concentration of A versus time, we can eliminate A).
- 00:58
Options C) and D) could be right if the reaction orders were different.
- 01:01
But, unfortunately, teachers don’t give credit for potential correctness. [Answers C and D crossed out]
- 01:05
So that means that a graph of B would give you a straight line for a first order reaction.
- 01:10
For first-order reactions, the graph of the natural log of the concentration of the reactant
- 01:15
versus time will be a straight line.
- 01:18
So B will take you straight to the finish line. [B crossing the finish line on a running track]
- 01:21
We’d tell you another chemistry pick up line, but all the good ones argon. [Periodic table and Argon asks to call me]
Related Videos
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?
AP Chemistry 3.2 Laws of Thermodynamics. What is the value for ΔG?