ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Laws of Thermodynamics Videos 15 videos
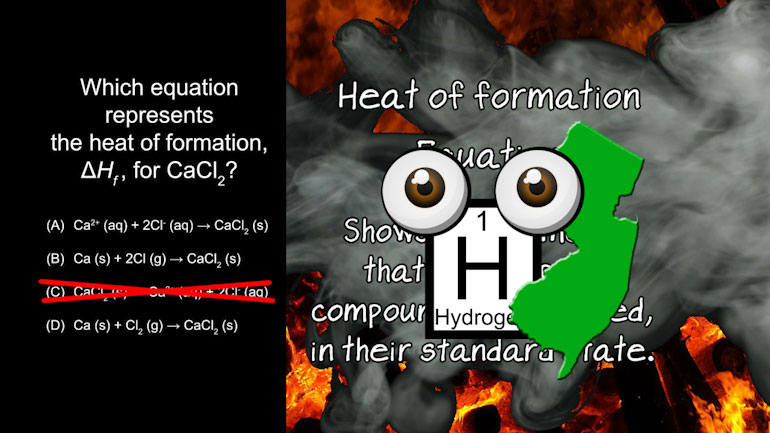
AP® Chemistry: Laws of Thermodynamics Drill 1, Problem 1. Which equation represents the heat of formation for Calcium Chloride?
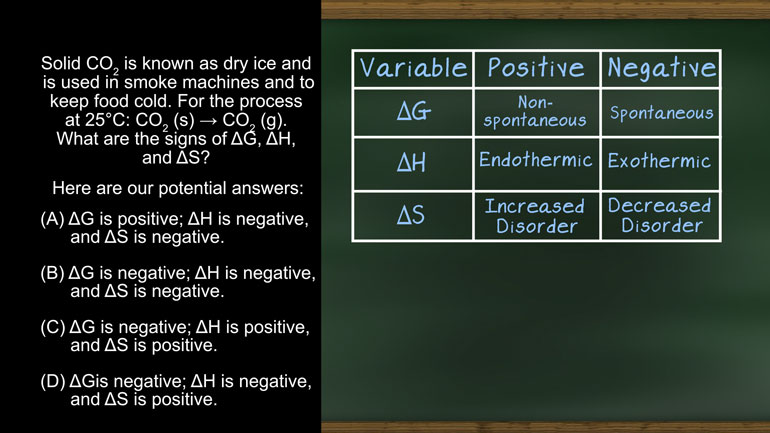
AP Chemistry 2.2 Laws of Thermodynamics. What is the ΔG and the spontaneity of the reacton?
AP Chemistry 2.5 Laws of Thermodynamics 8 Views
Share It!
Description:
AP Chemistry 2.5 Laws of Thermodynamics. Which of the following is true for a reaction at equilibrium?
Transcript
- 00:04
And here's your Shmoop du jour, brought to you by equilibrium, the human rights movement [People protesting outside a library]
- 00:08
that insists librarians should be treated equally.
- 00:12
Okay, here's our question.
- 00:14
Which of the following is true for a reaction at equilibrium?
- 00:19
And here are your potential answers.
Full Transcript
- 00:20
Okay, don’t get all “one with nature” on us here…we’re talking about molecular [Boy balancing books on his head]
- 00:26
equilibrium here.
- 00:28
That is, the point at which the forward rate of a reversible chemical reaction equals the
- 00:33
reverse rate. [Equilibrium of reaction depicted on a graph]
- 00:35
The concentrations of reactants and products don’t change, even though reactions are
- 00:38
still taking place.
- 00:41
So A and B make C and D at the same rate that C and D make A and B.
- 00:47
You thinking what we’re thinking? [Two guys jumping up and down]
- 00:48
Great, let's say it together.
- 00:50
3, 2, 1…Bananas!
- 00:53
…Oh.
- 00:54
Or example time.
- 00:55
That's good, too.
- 00:56
Okay, imagine that there’s a team of mathletes who decide that they should socialize more. [Mathletes socialising with each other]
- 01:03
So they schedule a hang-out sesh with the science Olympiad team studying next door.
- 01:07
We're sensing this is going to end in a rager. [Mathletes and Science Olympians together]
- 01:10
…Just us?
- 01:11
At some point, the students are split among the rooms, and the rate at which a mathlete
- 01:15
leaves his room is the same as the rate at which a different mathlete enters. [Mathletes leaving and entering the rooms]
- 01:21
The same is true for the science Olympians.
- 01:23
This smarty-swap can be thought of as our “reaction.”
- 01:27
The relative “concentration” of mathletes and science Olympians remains the same, despite
- 01:33
the fact that there’s still movement and different individuals might be present at [Olympians and Mathletes interchanging between rooms]
- 01:36
any given time.
- 01:38
And here we have achieved the ultimate nerdy equilibrium.
- 01:41
Hm.
- 01:42
Really thought it would end in a rager.
- 01:44
So let’s take another look at our question. [Boy using binoculars]
- 01:47
What do all these variables mean?
- 01:49
Well, DeltaH - or change in enthalpy - tells us if a reaction absorbs or releases
- 01:55
heat.
- 01:56
DeltaG, or the change in Gibbs free energy, tells us if a reaction is spontaneous
- 02:03
or non-spontaneous.
- 02:05
And DeltaS, or the change in entropy, tells us if a reaction leads to more or less [Delta H, G and S definitions]
- 02:10
disorder.
- 02:11
Of the science-y kind, not the mental kind. [Boy spinning a basketball on his finger]
- 02:14
Though too much chemistry will give the best of us anxiety.
- 02:17
Option A just means the reaction releases heat. [Girl cooking marshmallows on a fire]
- 02:19
It doesn’t tell us anything about equilibrium.
- 02:22
Option C tells us that there is no entropy change.
- 02:25
This just means that the degree of disorder remains the same.
- 02:29
Option D implies that there is no heat absorbed or released from the reaction.
- 02:32
All right, Option B. Not to Obi Wan you, but you’re our only hope. [Obi Wan appears and prods option B]
- 02:38
This option is all about spontaneity.
- 02:40
We know that if DeltaG<0, the reaction is spontaneous, and if DeltaG>0, the reaction is not spontaneous.
- 02:48
But what if DeltaG equals zero? [Boys walking a tight rope together]
- 02:50
This means the reaction is at equilibrium, and forward and reverse rates are the same.
- 02:54
So B is the right answer.
- 02:56
And there you have it folks, equilibrium.
- 02:57
Now let’s check on our smarty-pants party before somebody blows up a Bunsen burner. [Olympians and Mathletes socialising together in classrooms]
Related Videos
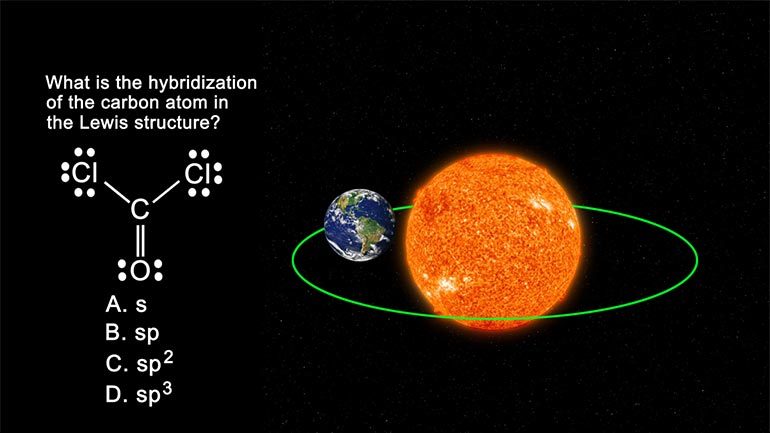
AP Chemistry: Structure of Atoms Drill 1, Problem 5. What is the hybridization of the carbon atom in the Lewis structure?
AP Chemistry DBQ/Free Response. Perform the following calculations.
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?