ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Laws of Thermodynamics Videos 15 videos
AP Chemistry 3.2 Laws of Thermodynamics. What is the value for ΔG?
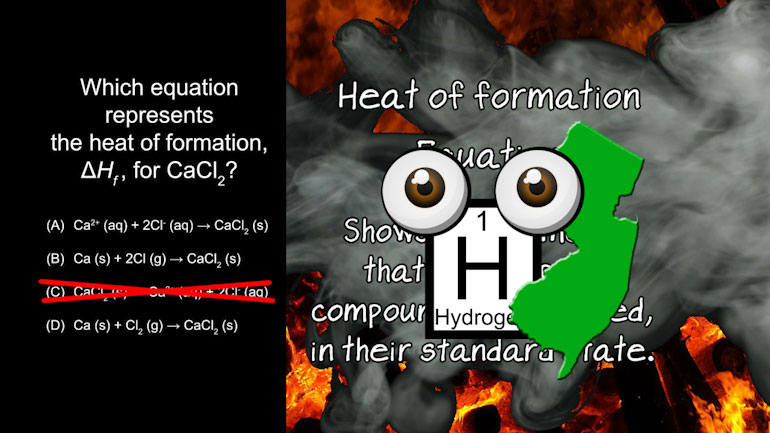
AP® Chemistry: Laws of Thermodynamics Drill 1, Problem 1. Which equation represents the heat of formation for Calcium Chloride?
AP Chemistry 2.3 Laws of Thermodynamics 6 Views
Share It!
Description:
This is a question about Gibbs free energy. You'd better act fast though. Otherwise, you may have to pay the normal sticker price of $99.99.
Transcript
- 00:03
And here's your Shmoop du jour, brought to you by free energy.
- 00:06
It’s like a free lunch, except, you know, it actually exists. [Girl approaches pizza store]
- 00:10
Okay, here's our question.
- 00:12
For ?G to have a negative value 100% of the time, ?H
- 00:18
and ?S should be which of the following, respectively?
Full Transcript
- 00:23
And here are our potential answers.
- 00:25
All right, well, the change in Gibbs free energy, or ?G, tells us whether
- 00:30
or not a chemical reaction is spontaneous. [Scientists working in a lab]
- 00:31
Not in the romantic way.
- 00:34
In the science way.
- 00:35
Though flowers are always a nice gesture…take notes, chemical reactions… [Scientist holding bunch of flowers]
- 00:40
If ?G is negative, the reaction is spontaneous.
- 00:44
If it’s positive, the reaction is not spontaneous and we have to add energy to get the reaction
- 00:49
to proceed. [Man holding glassware of chemical and goes up in flames]
- 00:50
We’re sure there’s a message about optimism in there somewhere.
- 00:54
Anyway, this question is asking us for the signs on ?H and ?S so that ?G is always negative,
- 01:02
and the reaction is always spontaneous.
- 01:05
So, what in the world are ?H and ?S, and what do they have to do with free energy?
- 01:09
No, it’s not hours and seconds, and no, free energy is not a sports drink. [Man holding energy on a track]
- 01:13
?H and ?S are thermodynamic quantities, and for this problem we care about how they relate
- 01:19
to ?G.
- 01:21
What we have to do is recall the Gibbs free energy equation.
- 01:24
We want to know when ?G is negative.
- 01:27
What do we have here?
- 01:28
This looks like a complex inequality. [Complex inequality example]
- 01:30
…No?
- 01:31
Not ringing a bell?
- 01:33
Go read up on complex inequalities. [Man reading a book]
- 01:35
We’ll be sitting here, waiting patiently.
- 01:38
Now let’s think about the necessary signs of ?H and ?S in order for this inequality
- 01:43
to always be true.
- 01:45
Add T?S to both sides to simplify the inequality.
- 01:47
Inequality – easy to simplify in science, not so much in life.
- 01:49
When using the Kelvin temperature scale, we know T is always positive. [temperature thermometer increases]
- 01:53
Multiplying by T won’t change the sign of the right hand side, so we can simply consider
- 01:58
the signs of ?H and ?S.
- 02:01
What signs on these variables make this inequality always true? [Girl standing with mathematical signs]
- 02:05
Let’s look at our options.
- 02:06
Let's check out A, for example.
- 02:07
If ?H and ?S are both positive, is the inequality always true?
- 02:12
If you can think of a scenario to disprove the inequality, then your answer is no.
- 02:17
The only scenario that can’t be disproven is choice (D).
- 02:21
The left hand side of the inequality is always negative, and the right hand side is always
- 02:26
positive, meaning the inequality is always true.
- 02:30
Because, you know, a positive number is always greater than a negative number. [Guys holding positive and negative number signs]
- 02:34
And there’s our message about optimism.
- 02:36
And now you know a little bit more about Gibbs free energy.
- 02:39
And if you happen to come across a free lunch, please… let us know. [People cue outside a store for free lunch]
Related Videos
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?
AP Chemistry 3.2 Laws of Thermodynamics. What is the value for ΔG?