ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
AP Chemistry Videos 45 videos
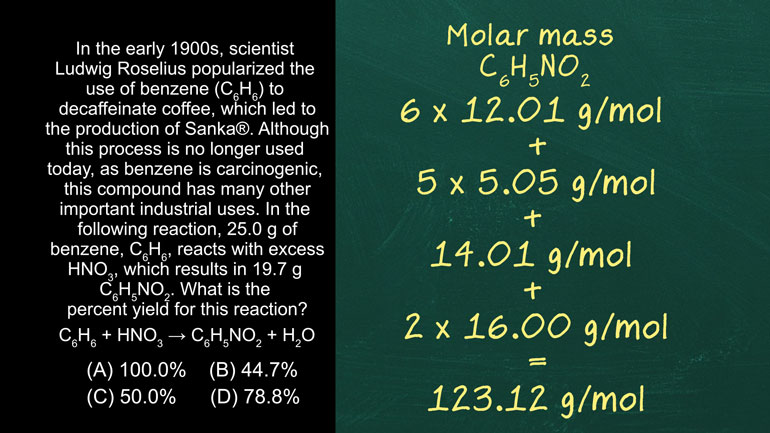
AP Chemistry 3.5 Chemical Reaction Rates. How much 85Kr will we need to collect?
Want to pull an Oliver Twist and ask us for more? We've gotcha covered. Head over to /video/subjects/test-prep/ap/ap-chemistry/ for more AP Ch...
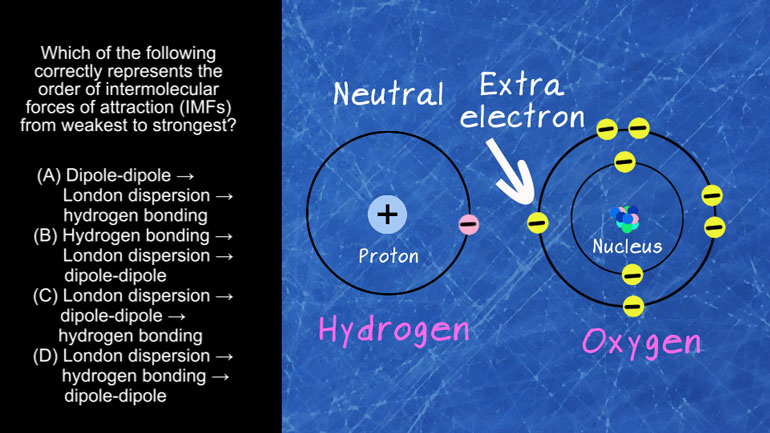
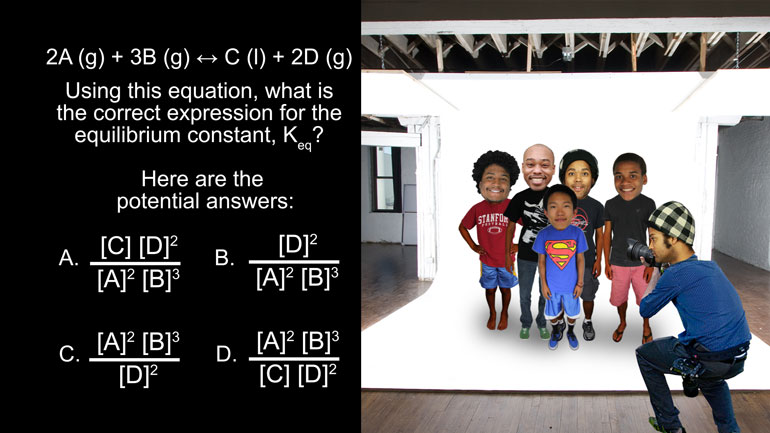
AP Chemistry 1.3 Forming and Breaking Bonds. Which of the following ions are spectator ions?
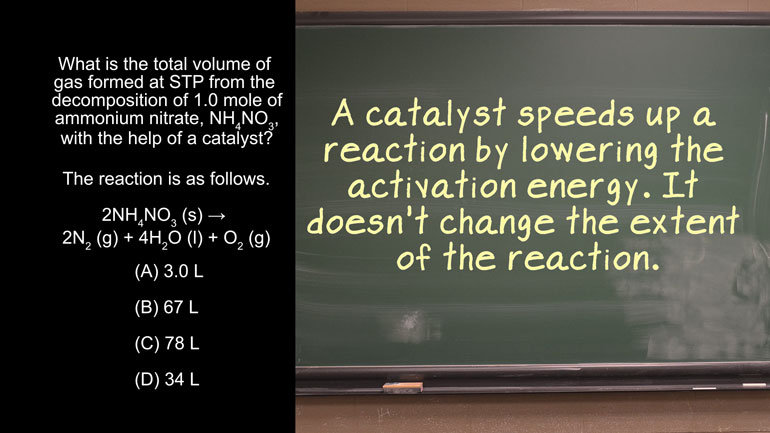
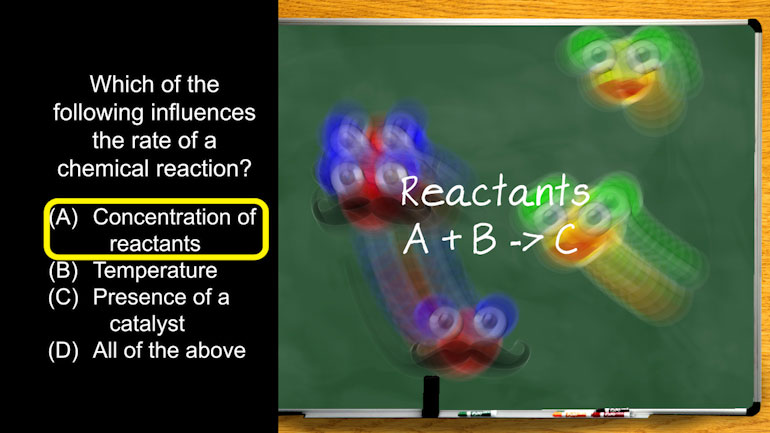
AP Chemistry 1.4 Chemical Reaction Rates 43 Views
Share It!
Description:
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
Transcript
- 00:00
Thank you We sneak and here's your shmoop du jour
- 00:05
Brought to you by units No not your great aunt
- 00:08
eunice units man your hearing's getting as bad as your
- 00:12
aunt units if rate is measured in mole's per liter
- 00:15
per second concentration is measured in miles per leader What
Full Transcript
- 00:19
is the correct units for a second order rate Constant
- 00:22
and your potential answers All right You know i get
- 00:24
asked that a lot of cocktail parties Okay well this
- 00:27
problems asking about the units for a rape constant but
- 00:29
there's a catch It doesn't give us the equation for
- 00:32
the rate law told so let's start by writing the
- 00:36
reaction rate law equation right there Rika rate law tells
- 00:40
us the reaction rate as a function of the rate
- 00:42
constant in the concentration of the reactant way don't know
- 00:46
what the reactant are but we do know that the
- 00:48
reaction is second order it's hoping to make the order
- 00:52
of the phoenix by the end of the year But
- 00:54
you don't have to put in it's time for us
- 00:56
Okay so the reaction order refers to the exponents on
- 00:58
the concentrations of the reacting species in the rate law
- 01:02
If we have only one reactant a then the concentration
- 01:06
of a must be squared in this equation Well the
- 01:08
question asked us to find the units on the rape
- 01:11
constant k Unfortunately we're given the units for the other
- 01:14
terms in the equation Good thing we wore our lucky
- 01:17
underwear today All right so here's the trick Since the
- 01:20
concentration of a is squared the units on a mole's
- 01:23
per leader are also squared After that all we have
- 01:27
to do to find the units on k is a
- 01:29
little math We have to isolate the unknown then group
- 01:34
the same units together and cancel out terms by term
- 01:39
correct units for the rate constant our leaders per mall
- 01:42
for second which is answer c Now how would you
- 01:46
rate that constant We'd give it five girl and meyer 00:01:48.966 --> [endTime] flasks
Related Videos
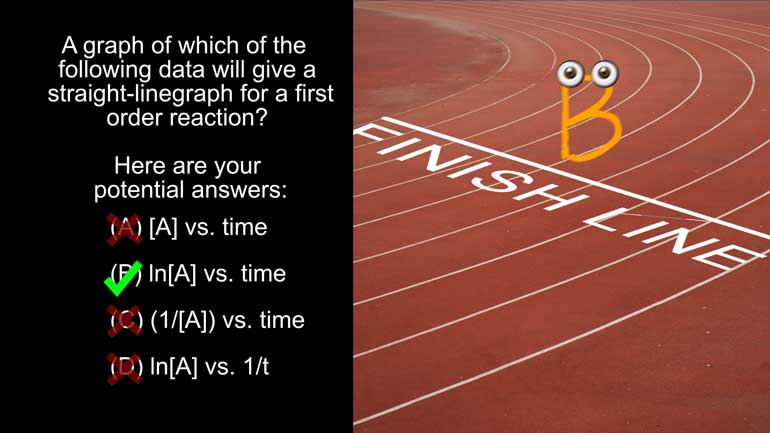
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?
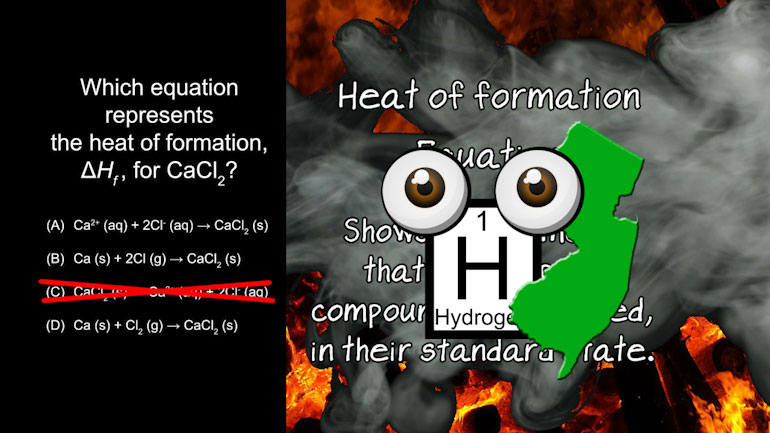
AP Chemistry 3.2 Laws of Thermodynamics. What is the value for ΔG?
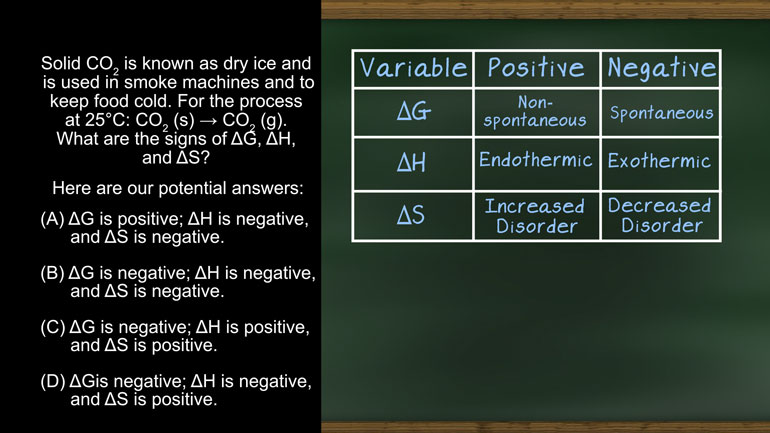
AP Chemistry 3.1 Laws of Thermodynamics. What is the change in enthalpy of this reaction?