ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
AP Chemistry Videos 45 videos
AP Chemistry 3.5 Chemical Reaction Rates. How much 85Kr will we need to collect?
Want to pull an Oliver Twist and ask us for more? We've gotcha covered. Head over to /video/subjects/test-prep/ap/ap-chemistry/ for more AP Ch...
AP Chemistry 1.3 Forming and Breaking Bonds. Which of the following ions are spectator ions?
AP Chemistry 1.3 Chemical Reaction Rates 189 Views
Share It!
Description:
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
Transcript
- 00:00
Thank you We sneak and here's your shmoop du jour
- 00:05
brought to you by the rate law rule that says
- 00:08
you can't give a movie more than five stars or
- 00:11
more than two comes up all right Suppose that the
- 00:14
reaction between species a and b has the rate law
Full Transcript
- 00:17
rate k a b squared what's the overall order of
- 00:20
the reaction and potential inches high own two three We
- 00:25
need to use the rate law to determine the reaction
- 00:27
border So let's look at this thing a little more
- 00:29
closely Rate law tells us how fast the chemical reaction
- 00:33
occurs K's the rate constant and beer the concentrations of
- 00:37
the reactant When it's like a kind of that memory
- 00:40
game right The blood more fun reaction order refers to
- 00:44
the exponents on the concentrations of the species In the
- 00:47
reaction we can look at the rate law to find
- 00:49
the reaction order with respect to each individual reactant as
- 00:52
well as for the overall reaction we'll hear The construction
- 00:56
of bee is raised in second tower and that means
- 00:58
the reaction is second order With respect to species be
- 01:02
we raise our own concentration We should be able to
- 01:04
See that there's no exponents written for species a which
- 01:08
means it's raised to the first power and the reaction
- 01:10
is first order With respect to species they will find
- 01:13
the overall reaction order We just add up the reaction
- 01:16
orders with respect to species a and b Last time
- 01:18
we checked one and two is three so the correct
- 01:21
answer is d i hope you'll give this video Six
- 01:24
stars Just keep it on the d l
Related Videos
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?
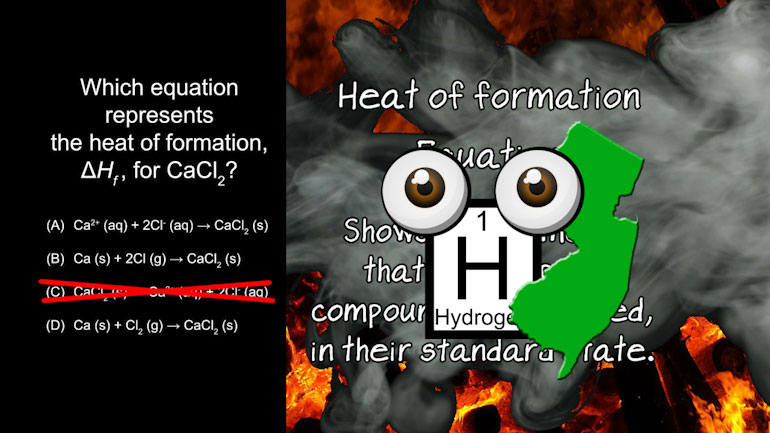
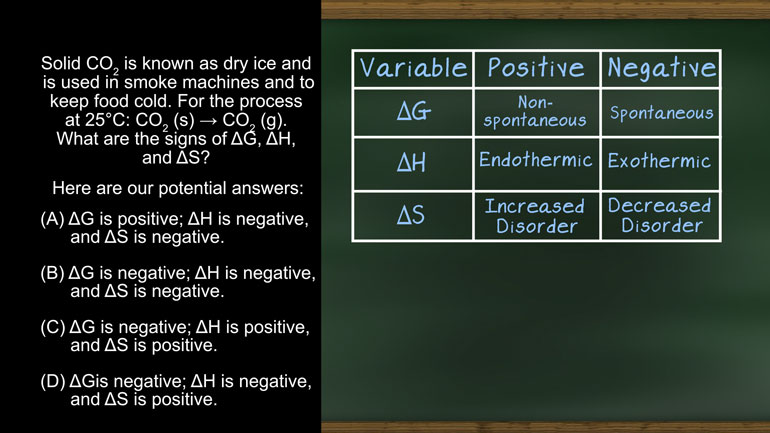
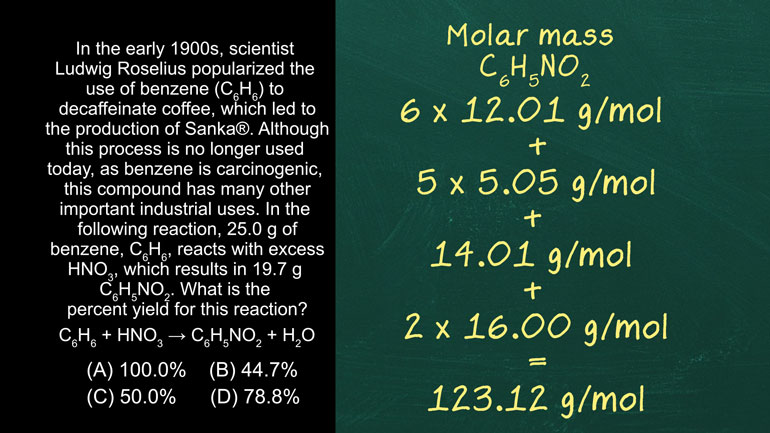
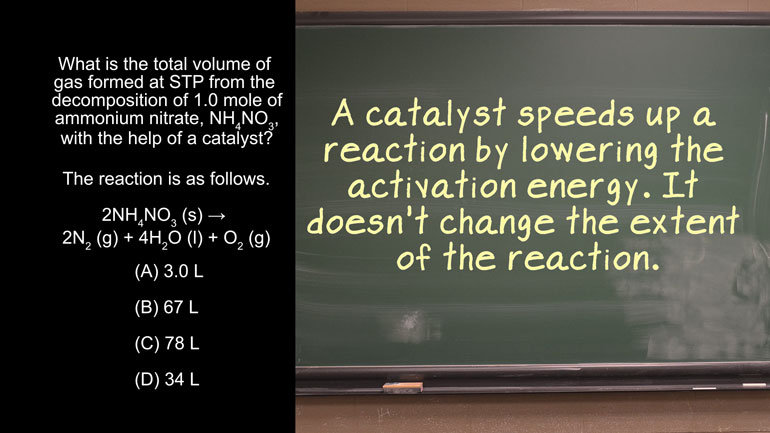
AP Chemistry 3.2 Laws of Thermodynamics. What is the value for ΔG?
AP Chemistry 3.1 Laws of Thermodynamics. What is the change in enthalpy of this reaction?