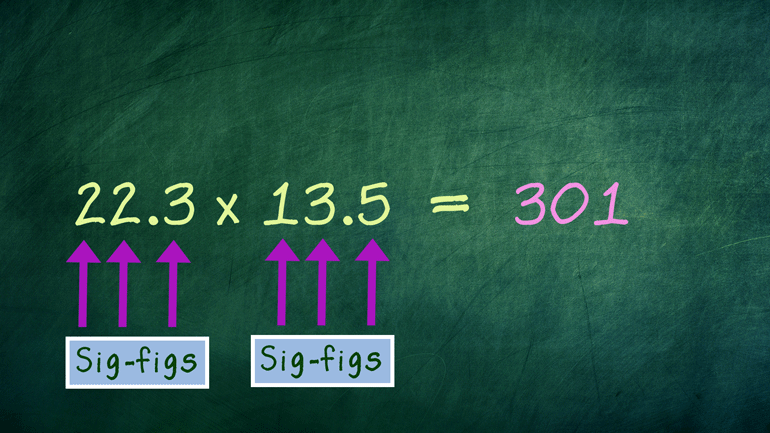ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Chemistry Videos 44 videos
Don't even think about stepping foot into a lab until you drink a nice big cup of safe-ty. Safety lesson number one: do not listen to this descript...
Today we're playing with fire. Wait, we're not supposed to say playing...having fun with fire? Today's lesson is on the colors that can be emitted...
These figures may not be significant to you, but they matter to us, okay? Oh, and to Science. They matter a ton to Science.
Chemistry: 5.8 Periodic Trends 120 Views
Share It!
Description:
Trends come and go periodically, but this periodic trend is here to stay. Forever. How is that possible? Check out the video to find out.
Transcript
- 00:03
Meet Yolanda. Yolanda likes
- 00:06
things done a certain way. It goes beyond [Yolanda waving]
- 00:08
just needing every hair on her head to
- 00:11
be perfectly in place or insisting on [Yolanda combing her hair]
- 00:13
wrinkle-free clothing. Everything on her [Yolanda ironing]
Full Transcript
- 00:15
desk needs to be at precise 90 or 180
- 00:18
degree angles from the edges. The
- 00:21
thermostat must always be set at exactly [Yolanda adjusting the thermostat]
- 00:24
70 degrees she arranges the corn flakes
- 00:27
in her cereal bowl into fractal patterns.
- 00:29
Yeah... it's intense. Anyway, so it's no wonder
- 00:32
that she's picky about the way her
- 00:34
elements are organized. If her table of [Messy table of elements]
- 00:37
elements looked like this, she'd have a
- 00:39
complete and total freak out. Yeah, she's
- 00:42
got a few simple rules. First, all
- 00:44
elements must be organized by atomic
- 00:46
radius. Atomic radius, which is one half
- 00:50
the distance between the nuclei of two
- 00:52
atoms, is used to determine an atom's size. [The atomic radius is shown on an element]
- 00:56
When Yolanda arranges her elements,
- 00:58
she demands that the atomic radii decrease
- 01:01
from left to right and increase from top [The direction of the decrease and increase are shown by arrows]
- 01:04
to bottom, like so. Yolanda's second
- 01:06
major ask is that the elements are
- 01:08
organized according to [The rules being written on a checklist]
- 01:10
electronegativity. Here, she prefers--okay,
- 01:13
prefers may not be a forceful enough word-- [Yolanda with her arms crossed]
- 01:16
that electronegativity, or an atom's
- 01:19
ability to attract an electron, increases
- 01:21
from left to right and decreases from
- 01:24
top to bottom.
- 01:25
There, perfect. We should be good on all
- 01:27
this now right? But no, Yolanda's [Dog looking disappointed]
- 01:29
fussiness knows no bounds. Her next
- 01:32
requirement is that the elements'
- 01:33
ionization energy, or the amount of
- 01:36
energy needed to remove an electron from
- 01:38
an atom, increases from left to right and [The elements are shown organised by their ionization energy]
- 01:41
decreases from top to bottom.
- 01:43
Well great...what top-notch organization.
- 01:46
Doesn't look like it could be improved
- 01:47
upon even a smidge. [Yolanda's dog giving a paw]
- 01:49
Oh, but wait: Yolanda also has a thing about
- 01:52
electron affinity, which is the measure
- 01:55
of how much an atom "wants" another
- 01:57
electron. Are they as hungry for one as this guy, [Guy with 4 burgers in front of him]
- 02:00
or are they more the house salad type? [Guy sat with a plate of salad]
- 02:03
Well, Yolanda won't settle for any
- 02:05
organizational approach that doesn't have
- 02:06
elements increasing
- 02:08
to electron affinity from left to
- 02:11
right and decreasing from top to bottom.
- 02:13
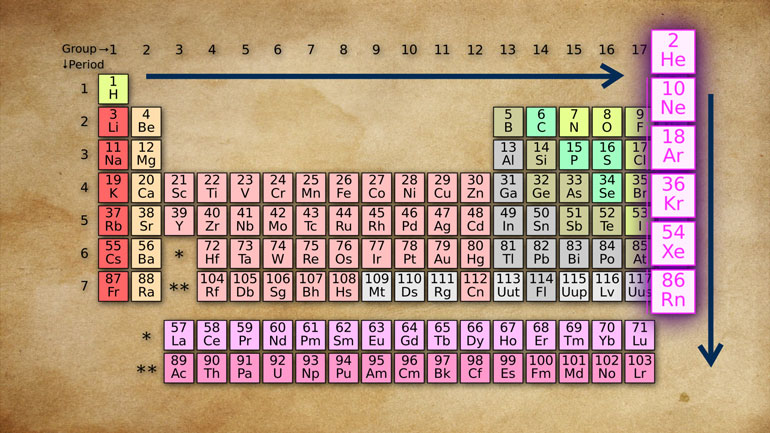
And wouldn't you know it? Yolanda's table looks [The periodic table is shown]
- 02:15
a bit like the periodic table. In fact, it
- 02:18
looks exactly like the periodic table,
- 02:20
which is because when the periodic table
- 02:22
was first put together in its current format, [Guy putting together the periodic table in segments]
- 02:24
it was assembled by an individual who
- 02:27
was as passionate as Yolanda about [Dmitri Mendeleev's dog saying "He never pets me."]
- 02:29
achieving a harmonious composition.
- 02:32
Unfortunately for our hero, she's going
- 02:34
to be a wee bit bummed when she finds
- 02:35
out that her submission for the Yolanda
- 02:38
table of the elements was denied by the
- 02:41
U.S. Patent Office. [Denied stamp on her application]
Related Videos
When you're about to marry the love of your life, not many things could stop you. However, finding out that your future hubby is keeping his crazy...
Here at Shmoop, we work for kids, not just the bottom line. Founded by David Siminoff and his wife Ellen Siminoff, Shmoop was originally conceived...
ACT Math: Elementary Algebra Drill 4, Problem 5. What is the solution to the problem shown?
AP® English Literature and Composition Passage Drill 1, Problem 1. Which literary device is used in lines 31 to 37?
AP® English Literature and Composition Passage Drill 2, Problem 1. What claim does Bacon make that contradicts the maxim "Whatsoever is delig...