ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Physical Science Videos 21 videos
ASVAB Physical Science 2.3. Melting is the process through which...what?
ASVAB Physical Science 2.4. A substance that consists of two or more things that are physically mixed together is a...what?
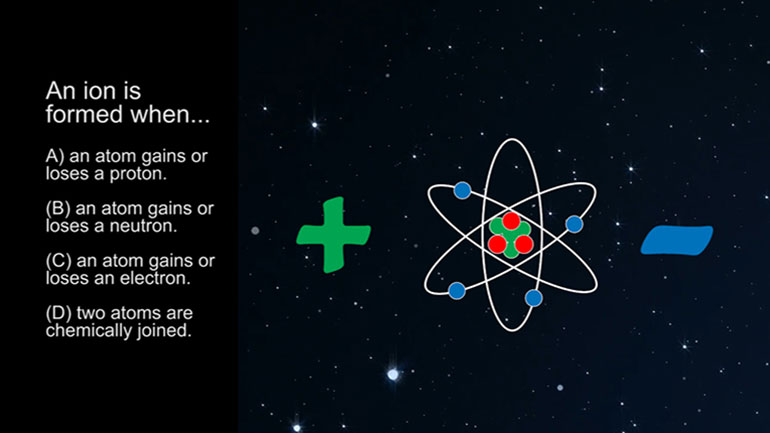
ASVAB Physical Science 3.1. Solution A has a pH of 3, while Solution B has a pH of 8. Solution A is what?
ASVAB Physical Science 3.1 192 Views
Share It!
Description:
ASVAB Physical Science 3.1. Solution A has a pH of 3, while Solution B has a pH of 8. Solution A is what?
Transcript
- 00:00
[ musical flourish ]
- 00:03
And here's your Shmoop du jour, brought to you by homemade volcanoes.
- 00:08
We prefer homemade cookies, but we'll settle for science projects instead.
- 00:12
All right, solution A has a pH of 3, while solution B has a pH of 8.
- 00:15
Solution A is what?
Full Transcript
- 00:17
And here are the potential answers.
- 00:18
[ mumbles ]
- 00:21
All right. Well, this is asking, uh,
- 00:24
what is a pH? Hmm? And how do you spell it?
- 00:27
Well, first, it's a measure of acidity
- 00:29
and, second, it's also kind of weird.
- 00:32
The pH scale runs from zero to 14.
- 00:34
The lower the number, the more acidic the substance is.
- 00:38
The higher the number, the more base it is.
- 00:40
If a substance has a pH of seven, like
- 00:43
plain water does, well, it means it's completely neutral.
- 00:46
Why couldn't the scale be one through ten or one through 20 or something simple? Hmm?
- 00:50
Well, sometimes scientists just like to make things confusing.
- 00:53
Consider it a perk of the job.
- 00:55
Well, here's one last tidbit of info before we get to the solution
- 00:58
or, uh, answer. We already have the solution.
- 01:00
See what we did there?
- 01:02
A lower pH means the solution has a higher concentration
- 01:05
of H+ ions.
- 01:07
And a higher concentration of H+ ions
- 01:09
means the solution is more acidic.
- 01:12
Now, onto the answer.
- 01:13
If the solution A has a pH of three, it's acidic.
- 01:16
That alone is enough to eliminate options C and D. Good-bye.
- 01:20
And if solution B has a pH of eight, we know it's
- 01:24
basic. Because a pH of greater than seven is, well, basic.
- 01:27
So solution A is acidic and solution B is basic.
- 01:31
The correct answer is option B.
- 01:33
Acids and bases and bears, oh, my!
- 01:35
[ chuckles ] [ music ]
Related Videos
ASVAB Paragraph Comprehension 1.2 Summary. Which of the following best describes the purpose of this passage?
ASVAB Paragraph Comprehension 1.2 Vocabulary-In-Context. In this passage, the word "illustrious" most nearly means...what?
ASVAB Paragraph Comprehension 1.3 Vocabulary-In-Context. The word "preposterous" most nearly means what?
ASVAB Paragraph Comprehension 2.3 Summary. Which of the following best describes the main idea of this passage?
ASVAB Word Knowledge: Word Roots, Prefixes, and Suffixes Drill 1, Problem 1. Which of these words is closest in meaning to incoherent?