ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Structure and Arrangement of Atoms Videos 9 videos
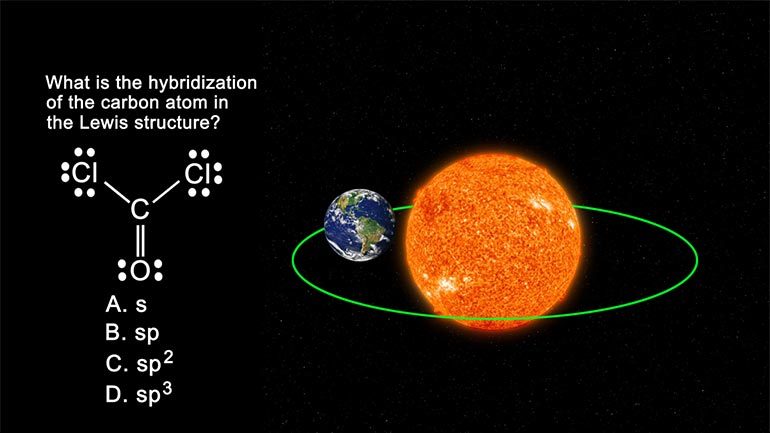
AP Chemistry: Structure of Atoms Drill 1, Problem 5. What is the hybridization of the carbon atom in the Lewis structure?
AP Chemistry 2.5 Structure and Arrangement of Atoms. Under which of the following conditions would a real gas behave more like an ideal gas?
AP Chemistry 3.2 Structure and Arrangement of Atoms 8 Views
Share It!
Description:
AP Chemistry 3.2 Structure and Arrangement of Atoms. What is the mole fraction of the solvent?
Transcript
- 00:04
Here’s your Shmoop du jour, brought to you by moles, helping you dig into chemistry the [A mole with two dinosaurs fighting]
- 00:09
dawn of time.
- 00:11
Let's burrow into the following question…
- 00:13
Fergie wanted to make lemonade from a mix that consisted primarily of sugar.
- 00:17
She prepared the lemonade solution by adding 0.50 mol of a sugar mix (solute) with 1.00
Full Transcript
- 00:23
mol of water (solvent).
- 00:26
What is the mole fraction of the solvent?
- 00:29
Here are the potential answers.. lot of numbers..
- 00:31
In order to satisfy Fergie’s craving, we're going to have to find some black eyed peas… [Black eyed peas stirring lemonade]
- 00:37
But if you just don't have those on hand, math will work just fine, too.
- 00:41
The question is asking us what mole fraction the solvent is.
- 00:43
So it might be helpful to know what mole fractions and solvents are before we dive in. [Lots of moles in the ground]
- 00:49
Well, the question conveniently tells us the solvent is water. [A girl drinking from a bottle of water]
- 00:52
If only all things in the wonderful world of AP-land were so easy…
- 00:56
And mole fraction doesn't refer to the unfortunate outcome of moles in The Texas Chainsaw Massacre… [A theatre showing the Texas Chainsaw Massacre on screen]
- 01:02
What the question actually means by mole fraction is simply what fraction of the total stuff
- 01:08
is the particular stuff we care about.
- 01:10
Kind of like if we were at a buffet that only offered two options: chocolate, [People holding plates at a buffet]
- 01:15
or asparagus-wrapped eggplant.
- 01:16
So what fraction of the buffet do we care about, or, what fraction of the buffet is
- 01:21
chocolate?
- 01:22
Also, where do we find a better buffet…?
- 01:24
Okay, back to the question…how much total stuff do we have? [Person sitting with feet up watching hoarders on TV]
- 01:28
Well, we have one mole of water and half a mole of sugar, so that gives us 1.50 total
- 01:32
moles of things in the mixture.
- 01:34
Since the question conveniently told us that the water is the solvent, we know that’s [A glass filling with water]
- 01:39
what we need to know the fraction of.
- 01:40
Now comes the scary part…
- 01:42
We're talking math.
- 01:43
But don't worry, it’s not that terrifying. [A kitten sat with a calculator]
- 01:45
All we have to do is divide the moles of the solvent by the total moles in the mixture.
- 01:49
We take 1.00 moles and divide it by 1.50 moles to give a mole fraction of 0.6666666666….
- 01:56
and let’s just round that to 0.667.
- 01:58
That means the answer is B.
- 02:00
But just for kicks and giggles, let's check out the rest of the answers. [Guy kicks friend in his leg]
- 02:03
Sounds almost as fun as chowing down at the chocolate, asparagus, eggplant buffet. [Man eating at a buffet and face turns green]
- 02:07
Since a mole fraction is some fraction of the total, it can’t be bigger than 1, which
- 02:11
rules out answer A.
- 02:12
Answer D, 0.500, says that half of the mixture would be water, which means there would be [A glass of water and a pile of sugar]
- 02:17
equal amounts water and sugar.
- 02:18
But if we look at the amounts we're given, 1 mole is more than 0.5 moles, so the fraction
- 02:24
should indicate that there is more water than there is sugar.
- 02:28
Answer D is out.
- 02:29
Similarly, answer C, 0.333, says that there is less water than sugar, which is also wrong.
- 02:35
Which leaves us with answer B again.
- 02:37
Phew. [Girl standing at a lemonade stand]
- 02:38
Time to wash that answer down with some refreshing lemonade and….
- 02:41
…Great.
- 02:42
All we have is eggplant and asparagus. [Eggplant and asparagus appear on a table of lemonade]
- 02:44
Seriously people, we need a new buffet around here c'mon.
Related Videos
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?
AP Chemistry 3.2 Laws of Thermodynamics. What is the value for ΔG?