ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Chemical Reaction Rates Videos 15 videos
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.1 Chemical Reaction Rates 323 Views
Share It!
Description:
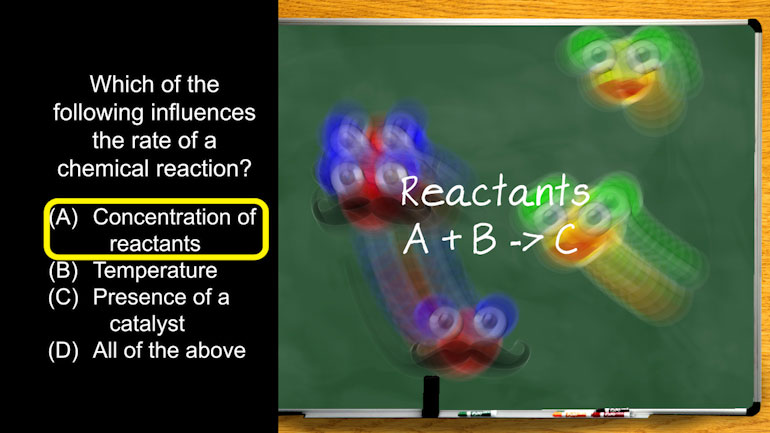
AP® Chemistry: Reaction Rates Drill 1, Problem 1. Which of the following influences the rate of a chemical reaction?
Transcript
- 00:03
Here's your shmoop du jour, brought to you by Catalysts.
- 00:06
Which are generally much more focused than Dogalysts.
- 00:17
Which of the following influences the rate of a chemical reaction?
- 00:25
So we're looking for what affects the rate of a chemical reaction. Other than, you know...
- 00:29
someone cracking a whip and ordering it to hurry the heck up.
Full Transcript
- 00:33
First, let's think about what a chemical reaction is. It's when two or more molecules react
- 00:39
with each other to form a different molecule.
- 00:46
Going even more granular, a reaction takes place when collisions take place between the
- 00:50
reactant particles to form a different arrangement of molecules.
- 00:56
Let's look at the first answer choice. Concentration of reactants.
- 01:01
Reactants are just a fancy name for the molecules that are reacting with each other.
- 01:05
The left side of a chemical equation.
- 01:08
Thinking logically, if we add more molecules
- 01:10
to a solution, because there are more molecules and hence more atoms to collide with each
- 01:15
other, the reaction will typically go faster. Yes, more collisions means faster.
- 01:21
So the concentration affects the rate. But before we just pick answer choice A, let's
- 01:28
keep looking. Answer choice B. The temperature. Temperature
- 01:32
is a measure of the average kinetic energy. Kinetic means movement, so in other words,
- 01:38
temperature affects the number of collisions that take place.
- 01:42
Higher temperatures would help speed up a reaction.
- 01:50
Therefore, Choice (B) is also a true statement.
- 01:52
We know that C must be true because the only
- 01:55
choices we have left are C and D...and answer choice D says ALL OF THE ABOVE.
- 02:00
But let's just take a look at C to be extra sure. The presence of a catalyst.
- 02:06
Well of course a catalyst will influence the speed, or rate, of a chemical reaction.
- 02:11
That's the point of a catalyst...to help the reactants form products faster. So again,
- 02:16
the rate is affected. Our answer's D.
- 02:19
As in, "Dogalyst..."
Related Videos
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?
AP Chemistry 3.2 Laws of Thermodynamics. What is the value for ΔG?